Neck Posture and Feeding Habits of Two Jurassic Sauropod Dinosaurs
Kent
A. Stevens
Computer and Information Science, University of Oregon, Eugene,
Oregon 9740
and
J. Michael Parrish
Biological Sciences, Northern Illinois
University, DeKalb, Illinois 60115
sciencemag.org version (with pdf download) is available here
AbstractArticulated digital reconstructions of two diplodocid sauropods revealed cervical poses and feeding envelopes. The necks of Diplodocus and Apatosaurus were nearly straight but gently declined such that the heads, which were themselves angled downwards relative to the neck, were close to ground level in their neutral, undeflected posture. Both necks were less flexible than conventionally depicted, and Diplodocus was less capable of lateral and dorsal curvature than Apatosaurus. The results suggest that these sauropods were best adapted to ground feeding or low browsing, contrary to the widely accepted model of diplodocid sauropods being high browsers. fauna of the Late Jurassic Morrison Formation was dominated by huge, long-necked sauropod dinosaurs. At least six genera occur in the Morrison, and five genera occur, apparently sympatrically, in the main quarry at Dinosaur National Monument (1,2). The abundance, size, and diversity of sauropods suggest that they played a central role in the Morrison and other continental ecosystems of the Jurassic and Cretaceous The fauna of the Late Jurassic Morrison Formation was dominated by huge, long-necked sauropod dinosaurs. At least six genera occur in the Morrison, and five genera occur, apparently sympatrically, in the main quarry at Dinosaur National Monument (1,2). The abundance, size, and diversity of sauropods suggest that they played a central role in the Morrison and other continental ecosystems of the Jurassic and Cretaceous. The key to understanding the feeding strategies of sauropods lies in the biomechanics of their elongate necks. When first described (3,4), Diplodocus was depicted with a neck that was nearly horizontal, or sloping down with a gentle dorsal arch (5,6). Recently, Diplodocus and other sauropods have been restored (7-9) with their heads far above ground level, with a sharp, swanlike dorsiflexion in the neck. Additionally, several taxa were depicted in a tripodal (hind limbs plus tail) pose (7,9). These inferences of near vertical necks in sauropods have sparked a lively debate (10-12) about how the sauropod circulatory system would have pumped blood to the elevated head that has yielded such creative suggestions as the presence of multiple hearts in the diplodocid Barosaurus (12). It is difficult to evaluate these alternative hypotheses by direct manipulation (13) of the original specimens because their fossil remains are too awkward, heavy, and fragile to move in articulation manually. In addition, postdepositional distortion often prevents proper articulation. As an alternative, we chose to manipulate detailed digital models of sauropod skeletons, with an interactive graphics software package (14) for constructing and articulating three-dimensional models of dinosaur skeletons. The entire axial skeleton and the major elements of the appendicular skeleton are rendered yet with dimensional accuracy. As the study concerns neck biomechanics, the geometry governing the mobility between each pair of cervical vertebrae was modeled in detail, with zygapophyseal facets reconstructed as complex, three-dimensional surfaces (Fig. 1) that were precisely positioned and oriented with respect to their associated centra by 24 adjustable parameters for each vertebra. This degree of modeling permitted reconstruction of the limits of deflection attainable by each neck vertebra. The posture for the trunk and limbs was based on previous reconstructions and not addressed by this study. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Figure 1. Closeup of tenth and eleventh cervical vertebrae in articulation, showing scaled, dimensionally accurate reconstructed zygapophyseal facets at the limit of dorsiflexion for Apatosaurus louisae (Carnegie Museum of Natural History specimen 3018).
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
We
modeled two well-known, Late Jurassic sauropod taxa, Apatosaurus and Diplodocus. These genera were selected because they were close
relatives (15-18), and were sympatric during the Late Jurassic (1,2). Both
genera are known from nearly complete skeletons (mounted Carnegie Museum of
Natural History [CM] specimens CM 3018 [Apatosaurus louisae]
and CM 84 [Diplodocus carnegii]), which share the same
number of neck vertebrae, and have shorter forelimbs than hindlimbs, yet differ
from one another significantly in build and vertebral form.
The
neutral pose and flexibility among cervical vertebrae was constrained by the
placement, size, and three-dimensional shape of their pre- and
postzygapophyses. The movement of
adjacent vertebrae, relative to the ball and socket articulations of the
centra, induces rotation and translation of the articulated pre- and
postzygapophyses. This movement places tension on the synovial capsule
surrounding each zygapophyseal pair. Our manipulation of muscle and ligament preparations of extant bird
necks indicated that synovial capsules constrain movement such that paired
pre-and postzygapophyses could only be displaced up to the point where the
margin of one facet reaches roughly the midpoint of the other facet, at which
point the capsule is stretched taut (19).
In other words, one facet could slip upon the other until their overlap
was reduced to approximately 50%. In vivo, muscles, ligaments, and fascia may
have further limited movement (19), thus the digital manipulations reported
here represent a "best case" scenario for neck mobility.
We measured the cervical vertebrae of CM 84 and CM 3018. The dimensions of
elements of the axial and appendicular skeletons were entered from tables in
the monographic descriptions of these specimens (3,4) and verified by our
photographs of the mounts. The 24 parameters for each of the 15 cervical
vertebrae in each specimen were entered and graphed. The few outliers or discontinuities were examined for
possible postmortem distortion, and corrected by reference to the original
material and by comparison with values taken from neighboring vertebrae.
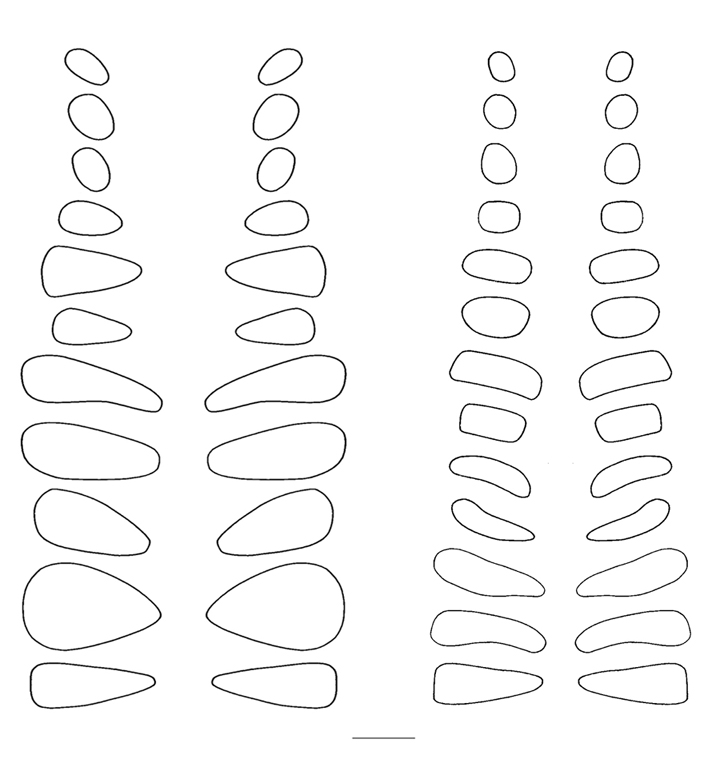
Finally, the margins of the articular facets of the zygapophyses, traced
directly from the specimens (Fig. 2), were used to bound the 3D shapes of their
digitally modeled counterparts.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Figure 2. Tracings of the articular facets of the prezygapophyses of Apatosaurus louisae (Carnegie Museum of Natural History specimen 3018), left, and Diplodocus carnegii (Carnegie Museum of Natural History specimen 84), right, taken from mounted specimens. Scale line represents 10 cm.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
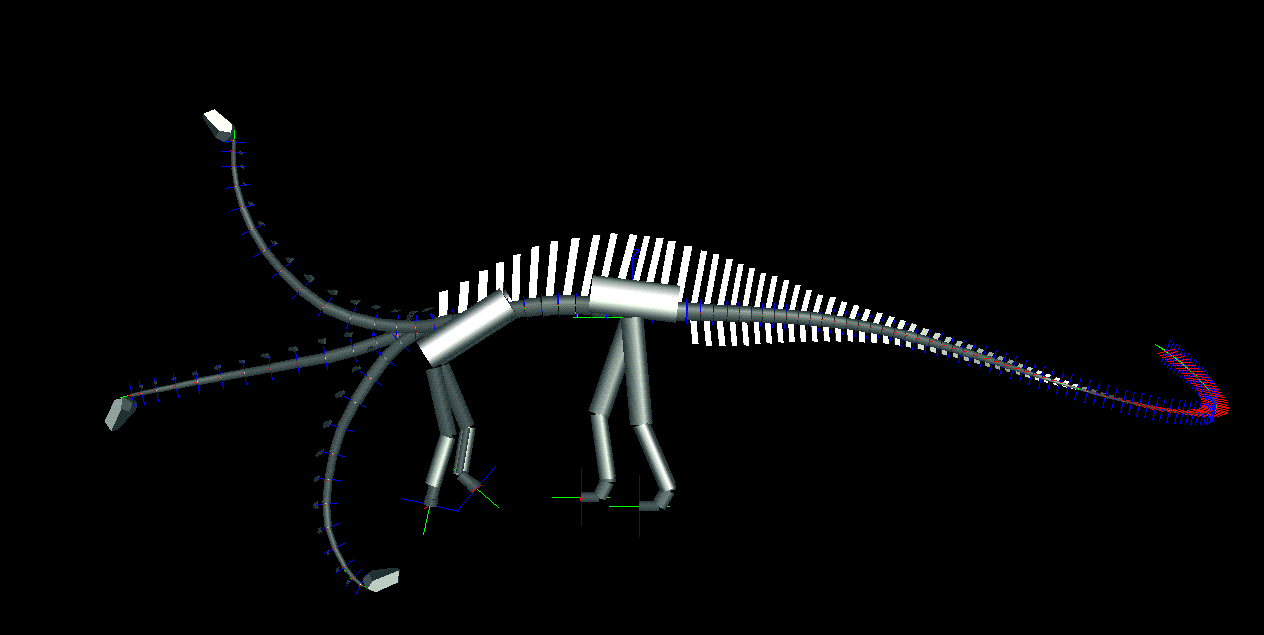
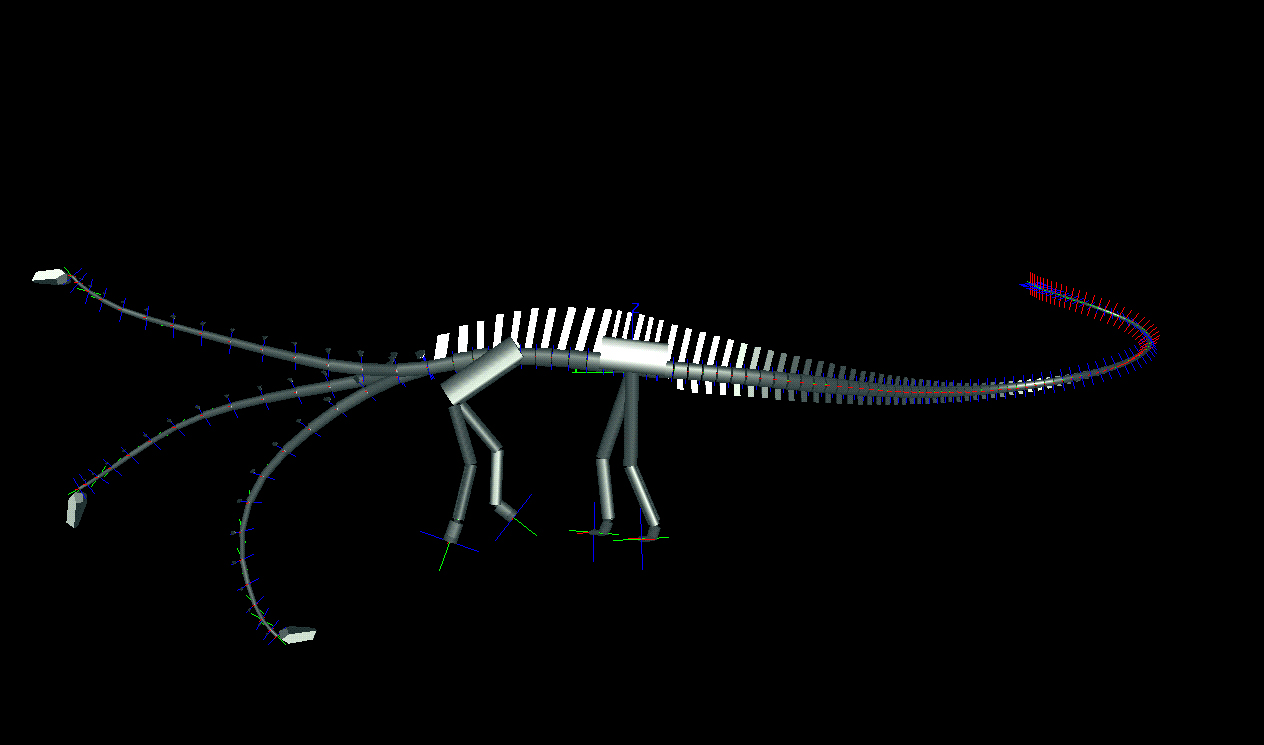
We determined the neutral poses for each animal, wherein the paired articular facets of the postzygapophyses of each cervical vertebra were centered over the facets of the prezygapophyses of its caudally adjacent counterpart. In this position, the ligaments and musculature controlling the neck's movement were relaxed, and the stresses on the joint capsules connecting the paired zygapophyses minimized. In the neutral pose, the necks of both diplodocid genera are remarkably straight, and the downward tilt of the spine at the shoulders brings the heads down to just above ground level (middle poses in Fig. 3). Diplodocids have "V"-shaped neural spines on their cervical vertebrae, which were probably attachment sites for parts of the nuchal ligament complex (20). Although living archosaurs do not have bifid spines, the presence of a compound nuchal ligament connecting the neural spines is common to birds and crocodilians (21, 22). At or near the neutral pose, such a nuchal ligament would be taut, and would hold the neck in its horizontal, cantilevered position without significant muscular exertion. In contrast, holding the neck in a raised position would have required continuous firing of the cervical dorsiflexors. The
posteroventral inclinations of the occipital condyles of Apatosaurus and Diplodocus (22-23) indicate that the heads were bent downwards in
a near-vertical orientation when in the neutral pose. The same condition is
observed in extant ground feeding ungulates. Moreover, the extreme dorsal placement of the orbits in both
genera was accompanied by an arcuate narrowing of the frontals between the
orbits that would have allowed effective anterior vision even when the head was
angled steeply downward, and perhaps permitted a useful degree of binocular
overlap ahead of the animal.
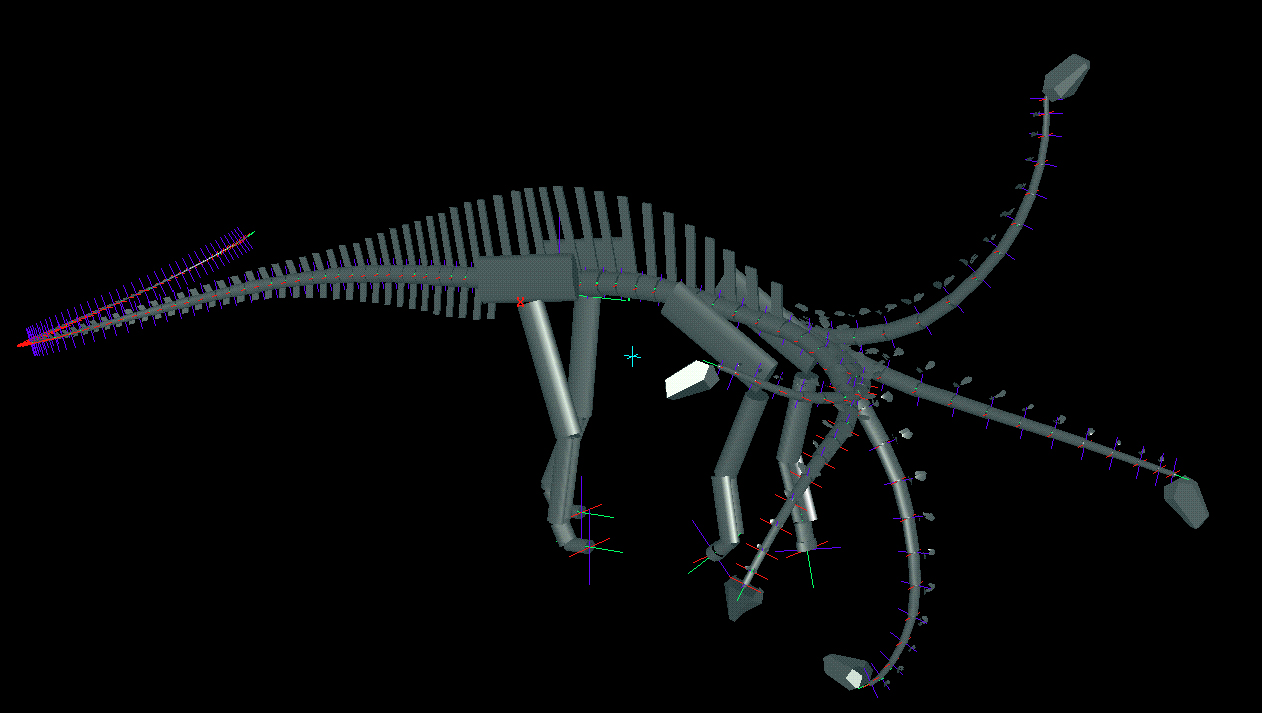
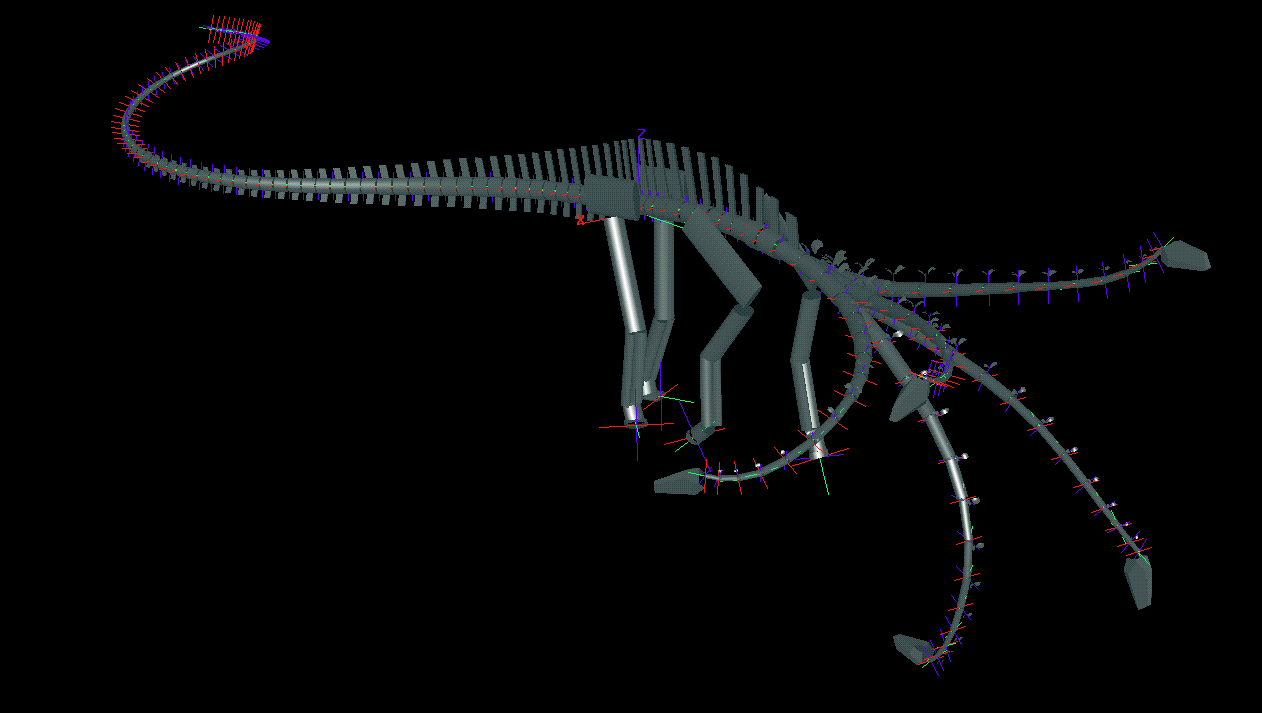
To determine the feeding envelope for each species, the neck was taken to maximum flexion laterally, then dorsally, ventrally, and subsequently in compound motion (see Figs. 3 and 4). In both lateral and vertical flexion, the most proximal cervicals created the greatest displacement from shoulder to head, producing in dorsal or lateral view an envelope of decreasing radius at the extremes of flexion. In anterior view, the envelope had greatest height sagittally, and greatest width in pure lateral flexion (compare tables 1 and 2). |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Figure 3. Lateral views of two sauropods in neutral pose and extremes of dorsoventral flexion as determined by DinoMorph of Apatosaurus louisae (modeled on Carnegie Museum of Natural History specimen 3018), top, and Diplodocus carnegii (modeled on Carnegie Museum of Natural History specimen 84), bottom.
Figure 4. Oblique views of Apatosaurus louisae (top) and Diplodocus carnegii (bottom) with superimposed images of extremes of dorsiflexion, ventriflexion, and low and high browsing with deflection to the right.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Table 1. The position of the atlantoccipital joint relative to origin at ground level below the base of the neck, for Apatosaurus louisae (Carnegie Museum of Natural History specimen 3018). Tables 1 and 2 show the position in 3D of the atlantoccipital joint (at the base of the cranium), where the origin of the coordinate system is at ground level directly below the base of the neck (below the C15/D1 joint) with the x axis positive to the right, y positive anteriorly, and z positive dorsally.
Table 2. The position of the atlantoccipital joint relative to origin at ground level below the base of the neck, for Diplodocus carnegii (Carnegie Museum of Natural History specimen 84)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Laterally, Apatosaurus could curl its neck to form a tight “U” curve, with its
head extended laterally over 4 m, owing to the large, flat zygapophyses of the
proximal cervicals. The zygapophyseal shape in Diplodocus permitted less angular deflection laterally, but the longer neck resulted in
similar total lateral deflection of about 4 m.
The primary difference between these two genera was in dorsiflexion. The maximum feeding height was about 6 m for Apatosaurus compared to 4 m for Diplodocus. The shorter yet more flexible neck of Apatosaurus could have achieved a pose close to the high “S” curve in which it is frequently depicted, while Diplodocus was barely able to elevate the head about the height of its back. The zygapophyseal design of Apatosaurus permitted greater decoupling between flexion in the horizontal and vertical planes. At the extremes of lateral flexion, Apatosaurus still had sufficient flexibility to feed from ground level up to more than 3 m, essentially squaring off the upper and lower corners of the feeding envelope. In contrast, lateral flexion of Diplodocus resulted in cumulative dorsiflexion distally, raising the head about 2 m above ground level in maximum lateral flexion, whereas the head of Apatosaurus could be at or near ground level with the same lateral flexion. Thus the feeding envelope of Apatosaurus, in anterior view, was subrectangular (both high and low feeding at the lateral extremes, whereas that of Diplodocus was more diamond-shaped, with less vertical range permitted at the lateral extremes and less overall elevation. Surprisingly, the necks of both genera were capable of more ventriflexion than dorsiflexion, reaching at least 1.5 m below ground level. The
low browsing envelopes of Diplodocus and Apatosaurus,
coupled with their similarities in skull shape and dentition (25), suggest that
these two sympatric giants may have been feeding on the same kinds of
vegetation. The abundance of both genera in Morrison deposits suggests that
either both were generalized feeders or that the plants upon which they fed
were so abundant that competition between the two genera was not a factor.
Despite the similarities in feeding envelopes and cranial morphology of the two
diplodocids, Diplodocus had a longer neck (6.2 m versus 5.3
m) and was more gracile in body shape and vertebral design than Apatosaurus,
which had roughly 3x the mass.
The extreme ventral flexibility in diplodocids is beyond the mobility required for quadrupedal feeding. Their ability to ventriflex sharply would have allowed a diplodocid on the margin of a lake or river to extend its head outwards and downwards to graze on plants on or under the water. Alternatively, some workers (7-9) have advocated tripodality in diplodocids, and a tripodal Diplodocus could have fed from 6.3-11.6 m off of the ground. However, the upper canopy arborescent plants available for a high feeding sauropod in the Late Jurassic consisted primarily of conifers and gingkoes (26-29), neither of which would provide particularly nutritious forage for large herbivores like diplodocids (30). On the other hand, an abundance of soft, high nutrition vegetation, in the form of ferns, cycadeoids, seed ferns, horsetails, and algae was available to terrestrial sauropods feeding along the shores of perennial lakes and rivers (31), a scenario for which the great neck length and ability to flex ventrally and traverse a significant lateral arc would have been advantageous. Furthermore, the presence of a near-horizontal neck in diplodocids renders moot the problem of supplying blood to an elevated sauropod brain. Rather than flexing their necks like dinosaurian counterparts of giraffes or swans, appear to have fed more like giant, longnecked bovids. References and Notes 1.
J.S. McIntosh. Bull. Carnegie Mus. 18, pp. 1-67 (1981).
2.
A.R. Fiorillo. Contr. Geol. U. Wyoming 30,
pp. 149-156 (1994).
3.
J.B. Hatcher. Mem. Carnegie
Museum Nat. Hist. 1(1) (1901).
4.
C.W. Gilmore. Mem. Carnegie Mus. Nat. Hist. 11(4) (1936).
5.
C.R. Knight, Oil Painting for Hall of Vertebrate Fossils, Carnegie Museum of
Natural History (1906).
6.
J. Sibbick, in D.B. Norman The Illustrated Encyclopedia of Dinosaurs (Salamander Books, London, 1985), pp. 80-81.
7. R.T. Bakker. The Dinosaur Heresies (W.F. Morrow, New York, 1986), pp. 14, 192, 203, 269.
8.
G.R. Paul. Modern Geology 23.
pp. 179-217 (1998).
9.
G.R. Paul, in Dinosaurs Past and Present, S.J. Czerkas and E.C. Olson, Eds. (U.
Washington Press, 1987), vol. 2, pp. 29-33.
10.
L.A. Hohnke. Nature 244,
pp. 309-310 (1973).
11.
R.S. Seymour Nature 262,
pp. 207-208 (1976).
12.
D.S.J. Choy and P. Altman. Lancet 340,
pp. 534-536 (1992).
13.
J. Martin Occ. Papers Tyrell Mus. Palaeont. 3,
pp. 154-159 (1987).
14.
The DinoMorph software was developed at U. of Oregon Department of
Computer and Information Science
(http://www.cs.uoregon.edu/~kent/dinoMorph.html).
15. J.S. McIntosh. Sauropoda. In The Dinosauria, D.B. Weishampel, P. Dodson,
and H. Osmolska, Eds. (U. California Press, Berkeley, 1990), pp. 345-401.
16.
P. Upchurch. Phil. Trans. Roy. Soc. Lond (B) 349,
pp. 365-390 (1995).
17.
P. Upchurch. Zool. J. Linn. Soc. 124, pp. 43-103 (1998).
18. J.A. Wilson and P.C. Sereno. J. Vert. Paleont. Memoir 5, pp. 1-68 (1998).
19.
J.M. Parrish and K. Stevens, unpublished dissections.
20.
R.M. Alexander Zoological Journal of the Linnean Society 83,
pp. 1-25 (1985).
21. J.C. George and A.J. Berger. Avian Myology (Academic Press, New York,
22. E. Frey. Stuttgarter Beitrage Naturkunde 426, pp. 1-60 (1988). 23.
A.R. Fiorillo. Hist. Biol. 13 pp. 1-16 (1998).
24. K. Carpenter and V. Tidwell. Modern Geology 23, pp. 69-84 (1998). 25. D.S. Berman and J.S. McIntosh. Bull. Carn. Mus. Nat. Hist. 8, pp. 1-35. 26.
C.N. Miller. Annals. of the Missouri Botanic Gardens 74,
pp. 692-706 (1987).
27.
D.J. Chure, K, Carpenter, R. Litwin, S. Hasiotis, and E. Evanoff. Modern
Geology 23, pp.507-537.
28. R. J. Litwin, C.A. Turner, and F. Peterson. Modern Geology 22, pp 297-319. 29. S.R. Ash and W.D. Tidwell. Modern Geology 22, pp. 321-378 (1998). 30.
A recent study of plant accumulations from the Morrison Formation of Colorado
showed a significant component of what may be conifer cuticle in what they are
interpreting as large herbivore coprolites, which may have been made by
sauropods. K. Chin and J.I. Kirkland. Modern Geology 23,
pp. 249-276.
31. T.M. Demko and J.T. Parrish. Morrison Geology 22, pp. 23-296. Acknowledgements:This research was funded by two grants from the Dinosaur Society. For access to specimens, we are grateful to David Berman and Mary Dawson (Carnegie Museum of Natural History), Mark Norell (American Museum of Natural History), Ken Stadtman (Brigham Young University), Dan Chure (Dinosaur National Monument), Richard Stucky and Ken Carpenter (Denver Museum of Natural History), Jim Madsen (Dinolab), and Bill Simpson (Field Museum). We have had fruitful discussions with Ken Carpenter, Dan Chure, Brian Curtice, Jim Kirkland, Jim Madsen, John Martin, Jack McIntosh, and Greg Paul. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||